what happens to the rate law at very high and very low concentration of hydrogen?
Chapter 12. Kinetics
12.1 Chemical Reaction Rates
Learning Objectives
By the end of this section, you will be able to:
- Ascertain chemic reaction rate
- Derive rate expressions from the balanced equation for a given chemical reaction
- Calculate reaction rates from experimental data
A rate is a measure of how some holding varies with time. Speed is a familiar rate that expresses the distance traveled by an object in a given corporeality of time. Wage is a rate that represents the corporeality of money earned by a person working for a given amount of time. Likewise, the rate of a chemical reaction is a measure of how much reactant is consumed, or how much production is produced, by the reaction in a given amount of time.
The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the fourth dimension dependence of some belongings that tin can be related to reactant or product amounts. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in book or force per unit area. For reactions involving one or more colored substances, rates may be monitored via measurements of light absorption. For reactions involving aqueous electrolytes, rates may exist measured via changes in a solution'south conductivity.
For reactants and products in solution, their relative amounts (concentrations) are conveniently used for purposes of expressing reaction rates. If we measure the concentration of hydrogen peroxide, H2O2, in an aqueous solution, nosotros find that it changes slowly over time every bit the H2O2 decomposes, according to the equation:
[latex]two\text{H}_2\text{O}_2(aq)\;{\longrightarrow}\;2\text{H}_2\text{O}(l)\;+\;\text{O}_2(grand)[/latex]
The rate at which the hydrogen peroxide decomposes can be expressed in terms of the rate of change of its concentration, as shown here:
[latex]\begin{array}{r @{{}={}} 50} \text{rate\;of\;decomposition\;of\;H}_2\text{O}_2 & - \frac{\text{change\;in\;concentration\;of\;reactant}}{\text{fourth dimension\;interval}} \\[0.5em] & - \frac{[\text{H}_2\text{O}_2]_{t_2}\;-\;[\text{H}_2\text{O}_2]_{t_1}}{t_2\;-\;t_1} \\[0.5em] & - \frac{{\Delta}[\text{H}_2\text{O}_2]}{{\Delta}t} \finish{array}[/latex]
This mathematical representation of the change in species concentration over time is the rate expression for the reaction. The brackets indicate molar concentrations, and the symbol delta (Δ) indicates "change in." Thus, [latex][\text{H}_2\text{O}_2]_{t_1}[/latex] represents the molar concentration of hydrogen peroxide at some time t i; as well,[latex][\text{H}_2\text{O}_2]_{t_2}[/latex] represents the molar concentration of hydrogen peroxide at a later time t 2; and Δ[H2Oii] represents the change in tooth concentration of hydrogen peroxide during the time interval Δt (that is, t ii − t 1). Since the reactant concentration decreases as the reaction proceeds, Δ[HtwoO2] is a negative quantity; we place a negative sign in front of the expression because reaction rates are, by convention, positive quantities. Effigy one provides an example of data collected during the decomposition of H2O2.
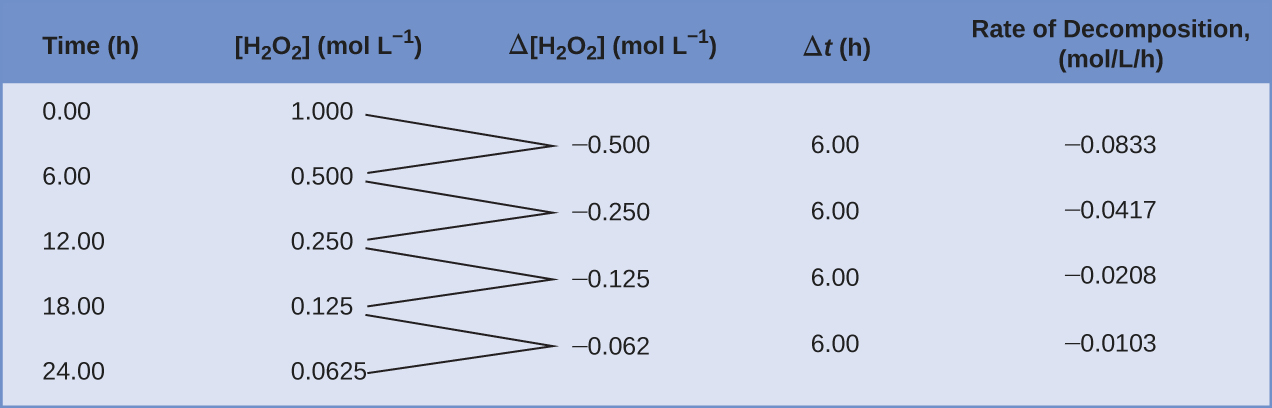
To obtain the tabulated results for this decomposition, the concentration of hydrogen peroxide was measured every 6 hours over the course of a day at a constant temperature of forty °C. Reaction rates were computed for each time interval by dividing the modify in concentration past the corresponding time increment, as shown hither for the showtime 6-hour catamenia:
[latex]\frac{-{\Delta}[\text{H}_2\text{O}_2]}{{\Delta}t} = \frac{-(0.500\;\text{mol/L}\;-\;1.000\;\text{mol/L})}{(6.00\;\text{h}\;-\;0.00\;\text{h})} = 0.0833\;\text{mol\;50}^{-1}\text{h}^{-1}[/latex]
Notice that the reaction rates vary with time, decreasing every bit the reaction gain. Results for the terminal 6-hr period yield a reaction rate of:
[latex]\frac{-{\Delta}[\text{H}_2\text{O}_2]}{{\Delta}t} = \frac{-(0.0625\;\text{mol/50}\;-\;0.125\;\text{mol/L})}{(24.00\;\text{h}\;-\;eighteen.00\;\text{h})} = 0.0104\;\text{mol\;L}^{-1}\text{h}^{-1}[/latex]
This behavior indicates the reaction continually slows with time. Using the concentrations at the beginning and end of a fourth dimension menses over which the reaction rate is irresolute results in the adding of an average charge per unit for the reaction over this time interval. At any specific time, the rate at which a reaction is proceeding is known as its instantaneous rate. The instantaneous rate of a reaction at "time zero," when the reaction commences, is its initial rate. Consider the analogy of a car slowing down every bit it approaches a end sign. The vehicle'south initial rate—analogous to the beginning of a chemic reaction—would be the speedometer reading at the moment the driver begins pressing the brakes (t 0). A few moments afterward, the instantaneous rate at a specific moment—telephone call information technology t ane—would be somewhat slower, as indicated by the speedometer reading at that signal in time. Equally time passes, the instantaneous charge per unit will continue to fall until information technology reaches zero, when the car (or reaction) stops. Unlike instantaneous speed, the motorcar'due south average speed is non indicated by the speedometer; but it can be calculated equally the ratio of the distance traveled to the time required to bring the vehicle to a complete end (Δt). Similar the decelerating machine, the average rate of a chemical reaction will fall somewhere between its initial and concluding rates.
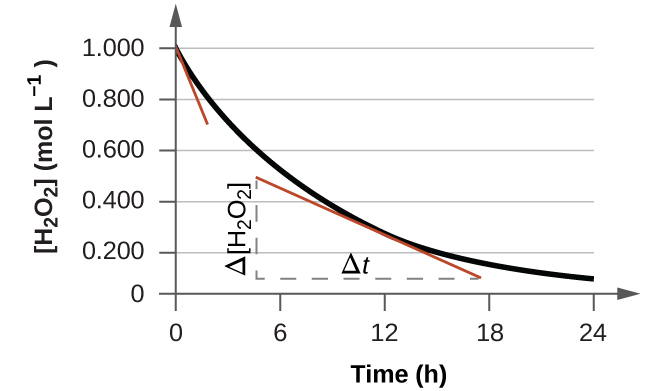
The instantaneous rate of a reaction may be adamant i of two ways. If experimental atmospheric condition allow the measurement of concentration changes over very brusque time intervals, then boilerplate rates computed as described earlier provide reasonably good approximations of instantaneous rates. Alternatively, a graphical procedure may exist used that, in issue, yields the results that would be obtained if brusk time interval measurements were possible. If we plot the concentration of hydrogen peroxide against time, the instantaneous rate of decomposition of H2O2 at whatsoever time t is given by the slope of a directly line that is tangent to the curve at that time (Effigy 2). We can use calculus to evaluating the slopes of such tangent lines, merely the process for doing so is beyond the scope of this chapter.
Reaction Rates in Analysis: Exam Strips for Urinalysis
Physicians often utilise dispensable test strips to measure out the amounts of various substances in a patient's urine (Figure 3). These test strips contain diverse chemical reagents, embedded in small pads at diverse locations along the strip, which undergo changes in color upon exposure to sufficient concentrations of specific substances. The usage instructions for test strips often stress that proper read time is disquisitional for optimal results. This accent on read fourth dimension suggests that kinetic aspects of the chemic reactions occurring on the examination strip are important considerations.
The exam for urinary glucose relies on a two-step procedure represented past the chemical equations shown here:
[latex]\text{C}_6\text{H}_{12}\text{O}_6\;+\;\text{O}_2\;{\xrightarrow[\text{catalyst}]{}}\;\text{C}_6\text{H}_{10}\text{O}_6\;+\;\text{H}_2\text{O}_2[/latex]
[latex]2\text{H}_2\text{O}_2\;+\;2\text{I}^{-}\;{\xrightarrow[\text{catalyst}]{}}\;\text{I}_2\;+\;two\text{H}_2\text{O}\;+\;\text{O}_2[/latex]
The first equation depicts the oxidation of glucose in the urine to yield glucolactone and hydrogen peroxide. The hydrogen peroxide produced subsequently oxidizes colorless iodide ion to yield brown iodine, which may be visually detected. Some strips include an additional substance that reacts with iodine to produce a more distinct colour modify.
The two test reactions shown above are inherently very ho-hum, just their rates are increased past special enzymes embedded in the examination strip pad. This is an example of catalysis, a topic discussed subsequently in this chapter. A typical glucose examination strip for use with urine requires approximately 30 seconds for completion of the colour-forming reactions. Reading the result too shortly might atomic number 82 one to conclude that the glucose concentration of the urine sample is lower than information technology actually is (a simulated-negative result). Waiting as well long to assess the color change can lead to a false positive due to the slower (non catalyzed) oxidation of iodide ion past other substances found in urine.

Relative Rates of Reaction
The charge per unit of a reaction may be expressed in terms of the change in the corporeality of any reactant or product, and may be but derived from the stoichiometry of the reaction. Consider the reaction represented by the post-obit equation:
[latex]2\text{NH}_3(g)\;{\longrightarrow}\;\text{North}_2(1000)\;+\;three\text{H}_2(g)[/latex]
The stoichiometric factors derived from this equation may be used to relate reaction rates in the same mode that they are used to related reactant and product amounts. The relation betwixt the reaction rates expressed in terms of nitrogen production and ammonia consumption, for example, is:
[latex]-\;\frac{{\Delta}\text{mol\;NH}_3}{{\Delta}t}\;\times\;\frac{ane\;\text{mol\;Northward}_2}{2\;\text{mol\;NH}_3} = \frac{{\Delta}\text{mol\;Due north}_2}{{\Delta}t}[/latex]
We can express this more simply without showing the stoichiometric factor'south units:
[latex]-\;\frac{one}{2}\;\frac{{\Delta}\text{mol\;NH}_3}{{\Delta}t} = \frac{{\Delta}\text{mol\;Northward}_2}{{\Delta}t}[/latex]
Note that a negative sign has been added to business relationship for the opposite signs of the two amount changes (the reactant amount is decreasing while the product amount is increasing). If the reactants and products are nowadays in the same solution, the molar amounts may be replaced by concentrations:
[latex]-\;\frac{1}{2}\;\frac{{\Delta}[\text{NH}_3]}{{\Delta}t} = \frac{{\Delta}[\text{N}_2]}{{\Delta}t}[/latex]
Similarly, the rate of formation of H2 is three times the rate of formation of Nii considering three moles of Htwo form during the fourth dimension required for the formation of one mole of Due north2:
[latex]\frac{1}{3}\;\frac{{\Delta}[\text{H}_2]}{{\Delta}t} = \frac{{\Delta}[\text{N}_2]}{{\Delta}t}[/latex]
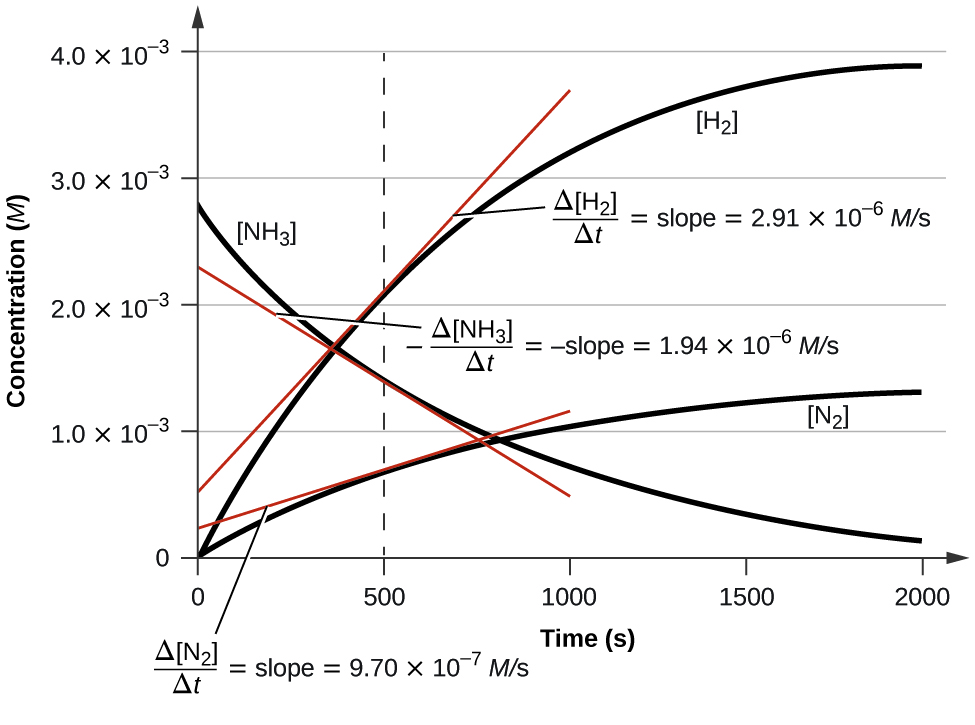
Figure 4 illustrates the change in concentrations over fourth dimension for the decomposition of ammonia into nitrogen and hydrogen at 1100 °C. We can run across from the slopes of the tangents drawn at t = 500 seconds that the instantaneous rates of change in the concentrations of the reactants and products are related past their stoichiometric factors. The rate of hydrogen product, for case, is observed to exist 3 times greater than that for nitrogen production:
[latex]\frac{2.91\;\times\;ten^{-6}\;M/\text{s}}{9.71\;\times\;10^{-6}\;Yard/\text{s}}\;{\approx}\;3[/latex]
Example 1
Expressions for Relative Reaction Rates
The starting time footstep in the product of nitric acid is the combustion of ammonia:
[latex]iv\text{NH}_3(thou)\;+\;v\text{O}_2(m)\;{\longrightarrow}\;four\text{NO}(g)\;+\;half-dozen\text{H}_2\text{O}(g)[/latex]
Write the equations that chronicle the rates of consumption of the reactants and the rates of germination of the products.
Solution
Because the stoichiometry of this homogeneous reaction, the rates for the consumption of reactants and germination of products are:
[latex]-\frac{1}{4}\;\frac{{\Delta}[\text{NH}_3]}{{\Delta}t} = -\frac{ane}{5}\;\frac{{\Delta}[\text{O}_2]}{{\Delta}t} = \frac{ane}{4}\;\frac{{\Delta}[\text{NO}]}{{\Delta}t} = \frac{1}{6}\;\frac{{\Delta}[\text{H}_2\text{O}]}{{\Delta}t}[/latex]
Bank check Your Learning
The rate of formation of Br2 is half-dozen.0 × 10−6 mol/50/s in a reaction described by the following net ionic equation:
[latex]5\text{Br}^{-}\;+\;\text{BrO}_3^{\;\;-}\;+\;vi\text{H}^{+}\;{\longrightarrow}\;iii\text{Br}_2\;+\;3\text{H}_2\text{O}[/latex]
Write the equations that relate the rates of consumption of the reactants and the rates of germination of the products.
Answer:
[latex]-\frac{1}{v}\;\frac{{\Delta}[\text{Br}^{-}]}{{\Delta}t} = -\frac{{\Delta}[\text{BrO}_3^{\;\;-}]}{{\Delta}t} = -\frac{ane}{6}\;\frac{{\Delta}[\text{H}^{+}]}{{\Delta}t} = \frac{1}{3}\;\frac{{\Delta}[\text{Br}_2]}{{\Delta}t} = \frac{i}{3}\;\frac{{\Delta}[\text{H}_2\text{O}]}{{\Delta}t}[/latex]
Instance ii
Reaction Rate Expressions for Decomposition of HiiO2
The graph in Figure two shows the rate of the decomposition of H2O2 over fourth dimension:
[latex]two\text{H}_2\text{O}_2\;{\longrightarrow}\;2\text{H}_2\text{O}\;+\;\text{O}_2[/latex]
Based on these data, the instantaneous rate of decomposition of HiiO2 at t = 11.1 h is determined to be
three.20 × 10−2 mol/L/h, that is:
[latex]-\frac{{\Delta}[\text{H}_2\text{O}_2]}{{\Delta}t} = 3.20\;\times\;10^{-2}\;\text{mol\;L}^{-one}\text{h}^{-1}[/latex]
What is the instantaneous charge per unit of production of H2O and O2?
Solution
Using the stoichiometry of the reaction, we may determine that:
[latex]-\frac{1}{two}\;\frac{{\Delta}[\text{H}_2\text{O}_2]}{{\Delta}t} = \frac{1}{2}\;\frac{{\Delta}[\text{H}_2\text{O}]}{{\Delta}t} = \frac{{\Delta}[\text{O}_2]}{{\Delta}t}[/latex]
Therefore:
[latex]\frac{1}{2}\;\times\;3.20\;\times\;x^{-2}\;\text{mol\;L}^{-1}\text{h}^{-1} = \frac{{\Delta}[\text{O}_2]}{{\Delta}t}[/latex]
and
[latex]\frac{{\Delta}[\text{O}_2]}{{\Delta}t} = 1.60\;\times\;10^{-2}\;\text{mol\;L}^{-ane}\text{h}^{-1}[/latex]
Check Your Learning
If the charge per unit of decomposition of ammonia, NH3, at 1150 K is 2.ten × 10−6 mol/Fifty/southward, what is the rate of product of nitrogen and hydrogen?
Answer:
ane.05 × 10−six mol/L/southward, Ntwo and 3.fifteen × 10−half-dozen mol/L/s, H2.
Primal Concepts and Summary
The rate of a reaction can be expressed either in terms of the subtract in the corporeality of a reactant or the increase in the corporeality of a product per unit fourth dimension. Relations betwixt different rate expressions for a given reaction are derived directly from the stoichiometric coefficients of the equation representing the reaction.
Central Equations
- relative reaction rates for [latex]a\text{A}\;{\longrightarrow}\;b\text{B} = -\frac{ane}{a}\;\frac{{\Delta}[\text{A}]}{{\Delta}t} = \frac{one}{b}\;\frac{{\Delta}[\text{B}]}{{\Delta}t}[/latex]
Chemistry End of Chapter Exercises
- What is the difference between boilerplate rate, initial rate, and instantaneous rate?
- Ozone decomposes to oxygen co-ordinate to the equation [latex]2\text{O}_3(g)\;{\longrightarrow}\;3\text{O}_2(m)[/latex]. Write the equation that relates the rate expressions for this reaction in terms of the disappearance of Oiii and the formation of oxygen.
- In the nuclear manufacture, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. Chlorine trifluoride is prepared by the reaction [latex]\text{Cl}_2(g)\;+\;iii\text{F}_2(yard)\;{\longrightarrow}\;2\text{ClF}_3(one thousand)[/latex]. Write the equation that relates the charge per unit expressions for this reaction in terms of the disappearance of Cl2 and F2 and the formation of ClFthree.
- A study of the charge per unit of dimerization of CivH6 gave the data shown in the table:
[latex]ii\text{C}_4\text{H}_6\;{\longrightarrow}\;\text{C}_8\text{H}_{12}[/latex]Fourth dimension (southward) 0 1600 3200 4800 6200 [CivHsix] (One thousand) i.00 × 10−2 5.04 × 10−3 3.37 × 10−3 2.53 × x−3 2.08 × 10−3 Table 1. (a) Decide the average charge per unit of dimerization betwixt 0 s and 1600 s, and between 1600 s and 3200 s.
(b) Estimate the instantaneous charge per unit of dimerization at 3200 s from a graph of time versus [CivH6]. What are the units of this charge per unit?
(c) Determine the average charge per unit of formation of C8H12 at 1600 southward and the instantaneous charge per unit of formation at 3200 s from the rates found in parts (a) and (b).
- A study of the rate of the reaction represented as [latex]2A\;{\longrightarrow}\;B[/latex] gave the following information:
Time (s) 0.0 5.0 10.0 xv.0 20.0 25.0 35.0 [A] (M) 1.00 0.952 0.625 0.465 0.370 0.308 0.230 Table two. (a) Decide the average rate of disappearance of A betwixt 0.0 southward and ten.0 s, and between ten.0 s and twenty.0 southward.
(b) Approximate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus [A]. What are the units of this rate?
(c) Use the rates plant in parts (a) and (b) to decide the average rate of germination of B between 0.00 s and 10.0 s, and the instantaneous charge per unit of formation of B at 15.0 south.
- Consider the following reaction in aqueous solution:
[latex]v\text{Br}^{-}(aq)\;+\;\text{BrO}_3^{\;\;-}(aq)\;+\;vi\text{H}^{+}(aq)\;{\longrightarrow}\;iii\text{Br}_2(aq)\;+\;3\text{H}_2\text{O}(fifty)[/latex]If the rate of disappearance of Br–(aq) at a particular moment during the reaction is 3.5 × 10−4 Chiliad due south −1, what is the rate of appearance of Br2(aq) at that moment?
Glossary
- average rate
- charge per unit of a chemical reaction computed as the ratio of a measured change in amount or concentration of substance to the time interval over which the alter occurred
- initial rate
- instantaneous rate of a chemical reaction at t = 0 s (immediately after the reaction has begun)
- instantaneous charge per unit
- charge per unit of a chemical reaction at whatsoever instant in time, determined past the slope of the line tangential to a graph of concentration as a function of time
- rate of reaction
- measure of the speed at which a chemic reaction takes place
- rate expression
- mathematical representation relating reaction rate to changes in amount, concentration, or pressure level of reactant or production species per unit fourth dimension
Solutions
Answers to Chemistry End of Chapter Exercises
one. The instantaneous rate is the rate of a reaction at any particular bespeak in time, a period of fourth dimension that is so short that the concentrations of reactants and products alter by a negligible corporeality. The initial rate is the instantaneous charge per unit of reaction as it starts (every bit product only begins to course). Average rate is the average of the instantaneous rates over a fourth dimension period.
3. [latex]\text{rate} = +\frac{ane}{2}\;\frac{{\Delta}[\text{CIF}_3]}{{\Delta}t} = -\frac{{\Delta}[\text{Cl}_2]}{{\Delta}t} = -\frac{i}{3}\;\frac{{\Delta}[\text{F}_2]}{{\Delta}t}[/latex]
5. (a) average rate, 0 − x s = 0.0375 mol Fifty−i s−1; boilerplate charge per unit, 12 − 18 s = 0.0225 mol 50−1 due south−1; (b) instantaneous rate, 15 south = 0.0500 mol Fifty−1 s−1; (c) average rate for B formation = 0.0188 mol 50−1 southward−1; instantaneous rate for B formation = 0.0250 mol L−1 due south−i
Source: https://opentextbc.ca/chemistry/chapter/12-1-chemical-reaction-rates/
0 Response to "what happens to the rate law at very high and very low concentration of hydrogen?"
Post a Comment